Abstract
Background: Myeloma patient outcomes remain heterogeneous, despite improving treatment options for newly diagnosed (NDMM) and relapsed/refractory (RRMM) disease. Improved individual risk prediction is highly desirable to support physicians and patients in increasingly complex joint decision-making processes. There is a specific need for readily accessible and easy to implement risk prediction tools that allow application in clinical trials and standard care. We previously reported on the utility of extended genetic profiling for refined outcome prediction in NDMM, including gain(1q), t(4;14) and del(17p) as a minimum set, to enable calling co-occurrence of two or more risk lesions, also termed double hit. To investigate its utility for prognostication in a wider context, we performed a meta-analysis of randomized clinical trials including in total 11150 patients with extended molecular profiling data for 5808 NDMM and RRMM patients.
Methods: This collaboration of the EMN, UNITO, HOVON, UK-MRA and GMMG academic study groups, to which industry contributed results following request, identified in total 14 prospective randomized interventional trials with availability of extended genetic profiles, i.e. complete information on risk markers gain(1q), t(4;14), del(17p) as minimum requirement, which were included in the analysis: nine trials for NDMM (four including transplant eligible (TE), three transplant ineligible (TNE), two both) and five trials for RRMM. Outcome analysis in relation to presence of no (no hit), one (single hit) or two or more (double hit) risk markers was performed for each trial individually using Cox proportional hazards models. Summary level meta-analysis was performed using the generic inverse-variance method in a random-effects model to synthesize hazard ratios (HR) for progression-free survival (PFS) and overall survival (OS). Heterogeneity was assessed using the I2 statistic and Cochran's Q test.
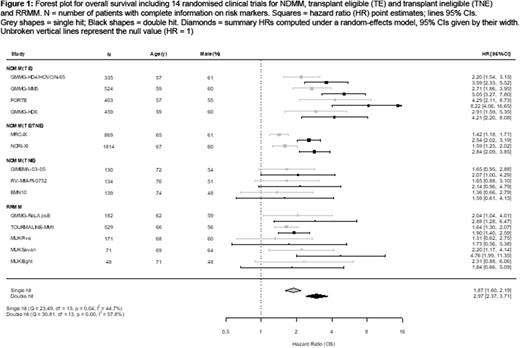
Results: In total 5808 trial patients were included in the analysis, 4807 NDMM patients (3296 transplant-eligible (TE), 1511 transplant-ineligible (TNE)), as well as 1001 patients with RRMM. Frequencies for presence of single (median: 38%; range 32%-48%) or double hit (14%; 9%-22%) were comparable between trials. OS was shortest for patients with double hit disease; meta-analysis summary HR for OS were 2.97 (95% CI: 2.37-3.71; P<0.0001) for double hit and 1.87 (95% CI: 1.60-2.19; P<0.0001) for single hit by meta-analysis (Figure 1). Summary HRs for PFS by meta-analysis were 2.18 (95% CI: 1.92-2.47; P<0.0001) for double hit and 1.63 (1.43-1.86; P<0.0001) for single hit. Estimated between-study heterogeneity was moderate (I2: 25%-59%). Results were consistent between NDMM and RRMM trials, and between trials with proteasome inhibitor (e.g. GMMG-HD4/HOVON-65; MUKFive) and immunomodulatory drug (IMiD) based regimens (e.g. GMMG-MM5; MUKSeven)(Figure 1). The association of double hit with shortened OS was most pronounced for trials with modern, intensive PI/IMiD induction/ASCT/maintenance treatments concepts, including FORTE (OS HR: 8.22; 95% CI: 4.06-16.65), GMMG-HD6 (OS HR 4.21; 2.20-8.08) and NCRI Myeloma XI (TE; OS HR: 3.65; 2.75-4.83). Hazard ratios were lower in trials for older, transplant-ineligible patients, but outcome was still consistently poorest for those patients with double hit MM.
Conclusions: This large meta-analysis demonstrates consistent risk-discrimination by extended genetic profiling in NDMM and, for the first time, RRMM. Our results strongly support assessment of and reporting on single hit/high risk and double hit/ultra-high risk patient groups in clinical trials, including trials for RRMM. These data also highlight the importance of access to extended genetic profiling in routine patient care, with the minimum requirement of obtaining a complete set of results for gain(1q), t(4;14) and del(17p) for all patients.
Disclosures
Kaiser:Seattle Genetics: Consultancy; Pfizer: Consultancy; Takeda: Honoraria; Janssen: Honoraria, Research Funding; BMS/Celgene: Honoraria, Research Funding; GSK: Consultancy; Karyopharm: Consultancy; AbbVie: Consultancy. Sonneveld:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Cairns:Takeda: Research Funding; Amgen: Research Funding; Celgene/BMS: Honoraria. Raab:Takeda: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Heidelberg Pharma: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Larocca:Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees. Brown:BMS/Celgene: Research Funding; Janssen: Research Funding. Li:Takeda: Current Employment. Mai:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses, Research Funding; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses, Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses, Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses, Research Funding. Cook:Amgen: Consultancy; BMS/Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Sanofi: Consultancy; Karyopharm: Consultancy. Goldschmidt:Molecular Partners: Research Funding; Merck Sharp and Dohme (MSD): Research Funding; Mundipharma GmbH: Research Funding; Takeda: Research Funding; Novartis: Honoraria, Research Funding; Adaptive Biotechnology: Consultancy; GlaxoSmithKline (GSK): Honoraria; Amgen, BMS, Celgene, Chugai, Dietmar-Hopp-Foundation, Janssen, Johns Hopkins University, Sanofi: Other: Grants and/or provision of Investigational Medicinal Product; Amgen, BMS, Celgene, Chugai, Janssen, Incyte, Molecular Partners, Merck Sharp and Dohme, Sanofi, Mundipharma GmbH, Takeda, Novartis: Research Funding; BMS: Consultancy, Honoraria, Other: Grants, Research Funding; Chugai: Honoraria, Other: grants, Research Funding; Janssen: Consultancy, Honoraria, Other: Grants, Research Funding; SANOFI: Consultancy, Honoraria, Other: Grants, Research Funding; Incyte: Research Funding; Amgen, BMS, Janssen, Sanofi, Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Other: Grants, Research Funding; AMGEN: Consultancy, Honoraria, Other: Grants, Research Funding; Amgen, BMS, Chugai, GlaxoSmithKline, Janssen, Novartis, Sanofi, Pfizer: Honoraria; Amgen, BMS, GlaxoSmithKline, Janssen, Novartis, Sanofi, Pfizer: Other: Support for attending meetings and/or travel; Array Biopharma: Research Funding; Dietmar-Hopp-Foundation: Research Funding. Boccadoro:AbbVie: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Research Funding; Celgene: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding. Jackson:BMS: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; J and |J: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Oncopeptides: Consultancy. Gay:Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; bluebird bio: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal